COUP-TFI/NR2F1 action on mitochondrial dynamics and hippocampal neuroplasticity: unveil novel etiopathogenetic mechanisms underlying intellectual disability
Dr. Sara Bonzano , post doc of the NICO research group of Adult Neurogenesis led by profs. Luca Bonfanti and Paolo Peretto, will be able to carry out the study in 2022 thanks to the post-doctoral grant awarded by the Interdisciplinary Center Linceo "Beniamino Segre" - in the field of Biomedicine.
Intellectual disability (ID) is a prevailing neurodevelopmental condition characterized by impaired cognitive abilities and severe deficits in the capability to adapt to the environment and social milieu. With a prevalence of 1–3% worldwide, these disorders represent a relevant medical and social challenge in our society.
A better understanding of the ID pathophysiology is needed for the development of new treatment strategies for these disorders and this goal will greatly benefit from novel discoveries on mouse models of ID.
In this context, studies on ID animal models have demonstrated defects in synaptic plasticity, a process required for sculpting modifications of the neural circuits in response to environmental changes, especially in brain areas involved in learning and memory (e.g. hippocampus). Interestingly, recent findings relying on in vitro observations have started pointing to the contribution of dysfunctional mitochondria in defective experience-evoked neuroplasticity. Nevertheless, whether and how mitochondrial dysfunctions are directly involved is still an open question and in vivo evidence is completely lacking.
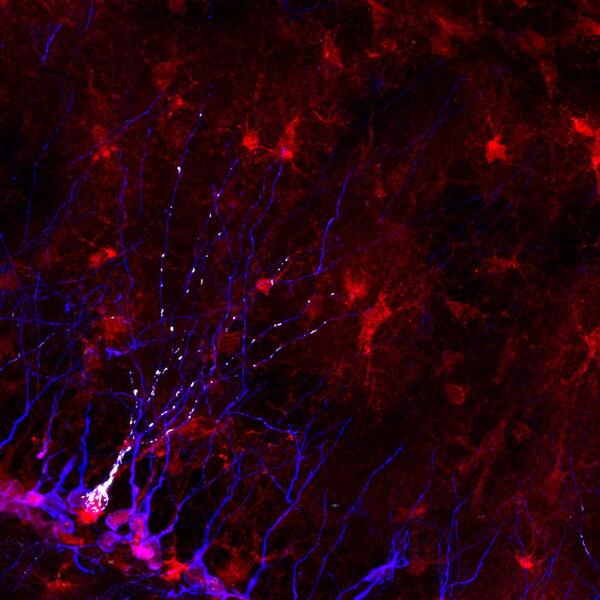
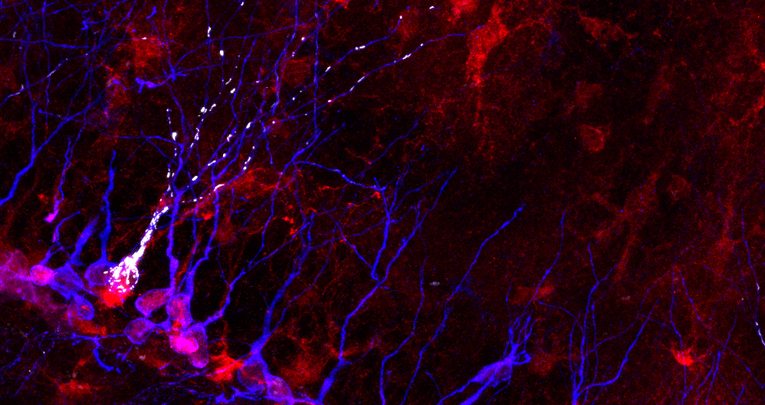
The hypothesis to be tested is if the disease gene COUP-TFI/NR2F1, whose mutations cause the rare ID-associated human disease BBSOAS, controls adaptive neuroplasticity by shaping mitochondrial network in newborn neurons of the postnatal mouse hippocampus.
By exploiting mouse genetics (i.e. COUP-TFI mutants) and multiple imaging tools, the project will focus on the challenging topic of mitochondrial dynamics and synaptic strengthening in the context of experience-driven neuroplasticity in dentate gyrus hippocampal neurons. Expected results will unveil novel mechanisms insights underlying cognitive skills and adaptive behavior and will uncover possible rescue strategies on mouse models of ID.