Glia
, 12 March 2020
In vivo silencing of miR-125a-3p promotes myelin repair in models of white matter demyelination
Marangon D 1 , Boda E 2,3 , Parolisi R 2,3 , Negri C 1 , Giorgi C 4 , Montarolo F 3,5 , Perga S 3,5 , Bertolotto A 3,5 , Buffo A 2,3 , Abbracchio MP 1 , Lecca D 1
Significance
Multiple Sclerosis (MS) is an autoimmune disease in which immune system cells enter the central nervous system and attack myelin on neuronal axons. Myelin can be repaired in the first phases of the pathology. In contrast, at chronic stages, myelin lesions accumulate and communication among neurons can be seriously affected. This can lead to motor, sensory and cognitive deficits.
Therapies currently in use aim at preventing MS relapses by restraining immune system activation. Therapies devoted to revert the “MS clock” by repairing the accumulated myelin lesion are still missing.
In a recent paper published on Glia journal, our researchers Enrica Boda, Roberta Parolisi, Annalisa Buffo ( Group of Physiopathology of Neural Stem Cells ), Francesca Montarolo, Simona Perga e Antonio Bertolotto (Group of Clinical Neurobiology), in collaboration with the research groups of Dr. Davide Lecca and Prof. Maria Pia Abbracchio ( University of Milan ) and of Dr. Corinna Giorgi (EBRI, Rome) , have demonstrated that silencing of the microRNA miR125a-3p results in a significant amelioration of myelin repair in MS animal models. This study provides an advancement in the comprehension of the mechanisms regulating myelin repair, and proposes a novel target potentially exploitable to design new therapeutic strategies for MS.
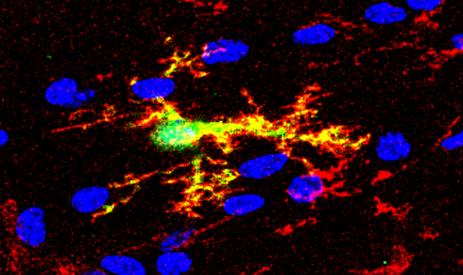
Oligodendrocyte progenitors overexpressing miR125a-3p (in green) maintain markers of immatury (i.e. NG2, in red)
Abstract
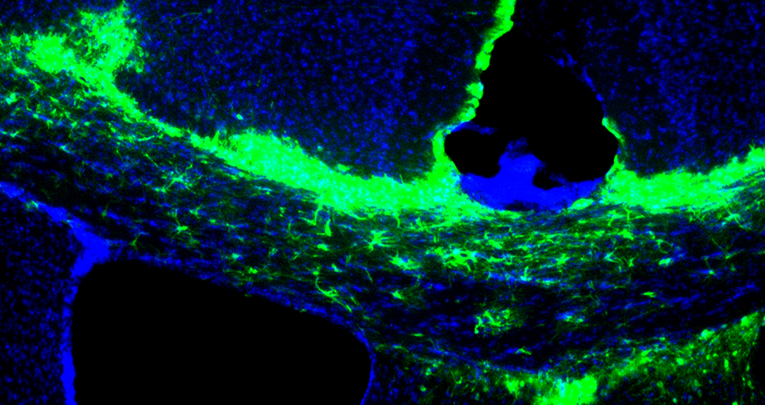
In the last decade, microRNAs have been increasingly recognized as key modulators of glial development. Recently, we identified miR-125a-3p as a new player in oligodendrocyte physiology, regulating in vitro differentiation of oligodendrocyte precursor cells (OPCs). Here, we show that miR-125a-3p is upregulated in active lesions of multiple sclerosis (MS) patients and in OPCs isolated from the spinal cord of chronic experimental autoimmune encephalomyelitis (EAE) mice, but not in those isolated from the spontaneously remyelinating corpus callosum of lysolecithin-treated mice.
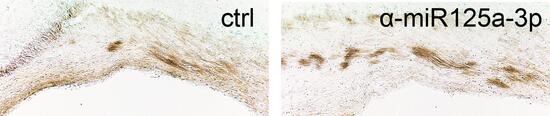
To test whether a sustained expression of miR-125a-3p in OPCs contribute to defective remyelination, we modulated miR-125a-3p expression in vivo and ex vivo after lysolecithin-induced demyelination. We found that lentiviral over-expression of miR-125a-3p impaired OPC maturation, whereas its downregulation accelerated remyelination. Transcriptome analysis and luciferase reporter assay revealed that these effects are partly mediated by the direct interaction of miR-125a-3p with Slc8a3, a sodium-calcium membrane transporter, and identified novel candidate targets, such as Gas7, that we demonstrated necessary to correctly address oligodendrocytes to terminal maturation.
These findings show that miR-125a-3p upregulation negatively affects OPC maturation in vivo, suggest its role in the pathogenesis of demyelinating diseases and unveil new targets for future promyelinating protective interventions.