Neuroendocrinology,
2020 June 22
G protein-couple destrogen receptor immunoreactivity in the rat hypothalamus is widely distributed in neurons, astrocytes and oligodendrocytes, fluctuates during the estrouscycle and is sexually dimorphic.
Marraudino M. a · Carrillo B. b · Bonaldo B. a · Llorente R. c · Campioli E. c · Garate I. d · Pinos H. b · Garcia-Segura L.M. e · Collado P. b · Grassi D. b,c,e
Introduction: The membrane-associated G protein-coupled estrogen receptor 1 (GPER) mediates the regulation by estradiol of arginine-vasopressin immunoreactivity in the supraoptic and paraventricular hypothalamic nuclei of female rats and is involved in the estrogenic control of hypothalamic regulated functions, such as food intake, sexual receptivity, and lordosis behavior.
Objective: To assess GPER distribution in the rat hypothalamus. Methods: GPER immunoreactivity was assessed in different anatomical subdivisions of five selected hypothalamic regions of young adult male and cycling female rats: the arcuate nucleus, the lateral hypothalamus, the paraventricular nucleus, the supraoptic nucleus, and the ventromedial hypothalamic nucleus. GPER immunoreactivity was colocalized with NeuN as a marker of mature neurons, GFAP as a marker of astrocytes, and CC1 as a marker of mature oligodendrocytes.
Results: GPER immunoreactivity was detected in hypothalamic neurons, astrocytes, and oligodendrocytes. Sex and regional differences and changes during the estrous cycle were detected in the total number of GPER-immunoreactive cells and in the proportion of neurons, astrocytes, and oligodendrocytes that were GPER-immunoreactive. Conclusions: These findings suggest that estrogenic regulation of hypothalamic function through GPER may be different in males and females and may fluctuate during the estrous cycle in females.
a
Department of Neuroscience “Rita Levi Montalcini,” Neuroscience Institute Cavalieri Ottolenghi, University of Turin, Turin, Italy
b
Department of Psychobiology, Universidad Nacional de Educación a Distancia, Madrid, Spain
c
Department of Preclinical Odontology, Universidad Europea de Madrid, Madrid, Spain
d
Department of Physiotherapy, Podology, and Dance, Universidad Europea de Madrid, Madrid, Spain
e
Instituto Cajal, CSIC, and Centro de Investigación Biomédica en Red Fragilidad y Envejecimiento Saludable, Instituto de Salud Carlos III, Madrid, Spain
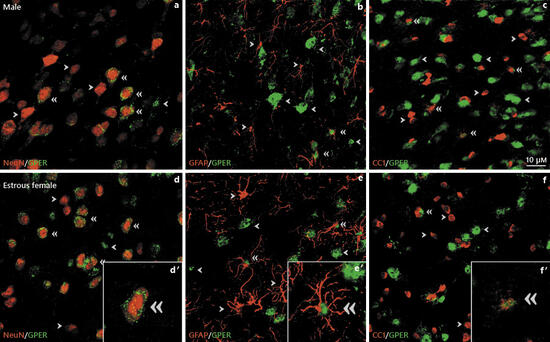
Representative examples of GPER colocalization with neuronal (NeuN) (a, d), astroglial (GFAP) (b, e), and oligodendroglial (CC1) (c, f) cellmarkers in the arcuate nucleus of the rathypothalamus of male (a–c) and estrousfemale (d–f) rats. Single arrow heads point to representative single-labeled cells. Double arrow heads point to representative double-labeled cells. Insets show examples of double-labeledcellsat high magnification. Scale bar, 10 µm. GPER, G protein-coupledestrogenreceptor 1.
Frontiers in Endocrinology, 2020 August 7
G Protein-Coupled Estrogen Receptor Immunoreactivity Fluctuates During the Estrous Cycle and Show Sex Differences in the Amygdala and Dorsal Hippocampus.
Ricardo Llorente 1 , Marilena Marraudino 2 , Beatriz Carrillo 3 4 , Brigitta Bonaldo 2 , Julia Simon-Areces 5 , Pedro Abellanas-Pérez 1 , Marina Rivero-Aguilar 1 , Jose M Fernandez-Garcia 3 4 , Helena Pinos 3 4 , Luis M Garcia-Segura 6 7 , Paloma Collado 3 4 , Daniela Grassi 1 3 4 6 7
G protein-coupled estrogen receptor (GPER) in the amygdala and the dorsal hippocampus mediates actions of estradiol on anxiety, social recognition and spatial memory. In addition, GPER participates in the estrogenic regulation of synaptic function in the amygdala and in the process of adult neurogenesis in the dentate gyrus. While the distribution of the canonical estrogen receptors α and β in the amygdala and dorsal hippocampus are well characterized, little is known about the regional distribution of GPER in these brain regions and whether this distribution is affected by sex or the stages of the estrous cycle.
In this study we performed a morphometric analysis of GPER immunoreactivity in the posterodorsal medial, anteroventral medial, basolateral, basomedial and central subdivisions of the amygdala and in all the histological layers of CA1 and the dentate gyrus of the dorsal hippocampal formation. The number of GPER immunoreactive cells was estimated in these different structures. GPER immunoreactivity was detected in all the assessed subdivisions of the amygdaloid nucleus and dorsal hippocampal formation. The number of GPER immunoreactive cells was higher in males than in estrus females in the central ( P = 0.001) and the posterodorsal medial amygdala ( P < 0.05); higher in males than in diestrus females in the strata orients ( P < 0.01) and radiatum-lacunosum-moleculare ( P < 0.05) of CA1-CA3 and in the molecular layer of the dentate gyrus ( P < 0.01); higher in diestrus females than in males in the basolateral amygdala ( P < 0.05); higher in diestrus females than in estrus females in the central ( P < 0.01), posterodorsal medial ( P < 0.01) and basolateral amygdala ( P < 0.01) and higher in estrus females than in diestrus females in the strata oriens ( P < 0.05) and radiatum-lacunosum-moleculare ( P < 0.05) of CA1-CA3 and in the molecular layer ( P < 0.05) and the hilus of the dentate gyrus ( P < 0.05). The findings suggest that estrogenic regulation of the amygdala and hippocampus through GPER may be different in males and in females and may fluctuate during the estrous cycle.