09/10/2017
Developmental Cell, October 2017
Anissa Kempf,
1,6,9
Enrica Boda,
2
Jessica C.F. Kwok,
3,8
Rafael Fritz,
4
Valentina Grande,
2
Andrea M. Kaelin,
1
Zorica Ristic,
1
Andre Schmandke,
1
Antonio Schmandke,
1
Bjoern Tews,
5
James W. Fawcett,
3
Olivier Pertz,
4,7
Annalisa Buffo,
2
and Martin E. Schwab
1
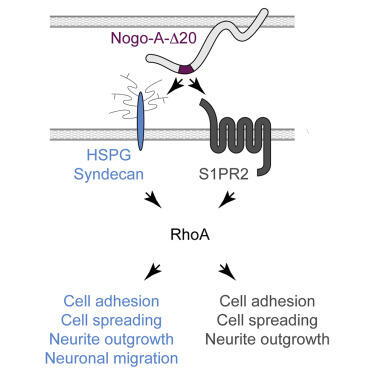
Heparan sulfate proteoglycans (HSPGs) critically modulate adhesion-, growth-, and migration-related processes. Here, we show that the transmembrane protein, Nogo-A, inhibits neurite outgrowth and cell spreading in neurons and Nogo-A-responsive cell lines via HSPGs.
The extracellular, active 180 amino acid Nogo-A region, named Nogo-A-Δ20, binds to heparin and brain-derived heparan sulfate glycosaminoglycans (GAGs) but not to the closely related chondroitin sulfate GAGs. HSPGs are required for Nogo-A-Δ20-induced inhibition of adhesion, cell spreading, and neurite outgrowth, as well as for RhoA activation.
Surprisingly, we show that Nogo-A-Δ20 can act via HSPGs independently of its receptor, Sphingosine-1-Phosphate receptor 2 (S1PR2). We thereby identify the HSPG family members syndecan-3 and syndecan-4 as functional receptors for Nogo-A-Δ20. Finally, we show in explant cultures
ex vivo
that Nogo-A-Δ20 promotes the migration of neuroblasts via HSPGs but not S1PR2.
>> read full article
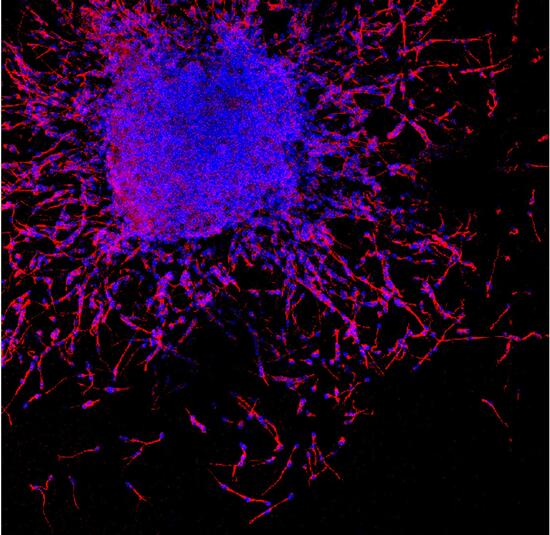
In this picture young neurons (in red) produced by neural stem cells migrate away from the mother cells.
In a first migratory phase, young neurons slide over each other thanks to repulsive signals mediated by the interaction between
NoGo-A and
Heparan Sulphate Proteoglycans
locate
on
the
cells' membrane.
NoGo-A is a membrane protein. Its tail (delta20 segment, in purple) interacts with Heparan Sulphate Proteoglycans (light blue) on the membrane of adjacent cells, thereby inducing diverse effects on brain plasticity.
1
Brain Research Institute, University of Zurich and Department of Health Sciences and Technology, Swiss Federal Institute of Technology (ETH) Zurich, 8057 Zurich, Switzerland
2
Department of Neuroscience, Neuroscience Institute Cavalieri Ottolenghi (NICO), Università degli Studi di Torino, Orbassano, Turin 10043, Italy
3
John van Geest Centre for Brain Repair, Department of Clinical Neurosciences, University of Cambridge, Robinson Way, Cambridge CB2 0PY, UK
4
Institute for Biochemistry and Genetics, Department of Biomedicine, University of Basel, 4058 Basel, Switzerland
5
Schaller Research Group at the University of Heidelberg and the German Cancer Research Center (DKFZ), Molecular Mechanisms of Tumor Invasion, 69120 Heidelberg, Germany
6
Present address: University of Oxford, Centre for Neural Circuits and Behaviour, Oxford OX1 3SR, UK
7
Present address: Institute of Cell Biology, University of Bern, Bern 3012, Switzerland
8
Present address: School of Biomedical Sciences, Faculty of Biological Sciences, University of Leeds, Leeds LS2 9JT, UK